Cardiovascular simulators – what is it simulated
The adoption of in-vitro cardiovascular simulators by the medical device industry driving current and future innovation. Companies have now access to customised in-vitro simulation technologies that help replicating highly complex cardiovascular scenarios such as stroke, coronary disease and aneurysm. Each of the customised in-vitro simulators developed by MET provide the anatomical challenges that a medical device has to overcome during its clinical use.
Cardiovascular simulators – their customisation
MET researchers work with the digital medical data provided by the latest Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) scanning technology. The clinical aspects are accurately extracted from the medical data and translated into engineering data by our dedicated team of specialists. The results are customised virtual models, on their true scale and clinical complexity. The virtual data are fed into the design and fabrication processes of the clinical relevant anatomical physical models. The customised models could be used as stand-alone in-vitro setups for device testing or as integrated parts of a customised in-vitro cardiovascular simulator.
How do cardiovascular simulators benefit medical devices development?
MET cardiovascular simulators provide non-clinical engineering testing services as recommended by the FDA such as delivery, deployment and retraction, pushability, flexibility and kink test, accuracy of deployment, vessel anchoring force before slippage, migration, ease of preparation, ease of introduction and radiopacity. MET in-vitro models mimic customised challenging in-vivo tortuous anatomy, vessel wall friction and mechanical behavior, which a device has to overcome in clinical use. The in-vivo physiologic and anatomic conditions are fully mimicked by our customised in-vitro simulators which are validated by clinicians associated with the centre.
Example of in-vitro simulators technologies informing medical device design
The Abdominal Aortic Aneurysm (AAA) simulation unit is one of MET customised in-vitro cardiovascular simulators, which was validated to offer multiple testing options for industry such as:
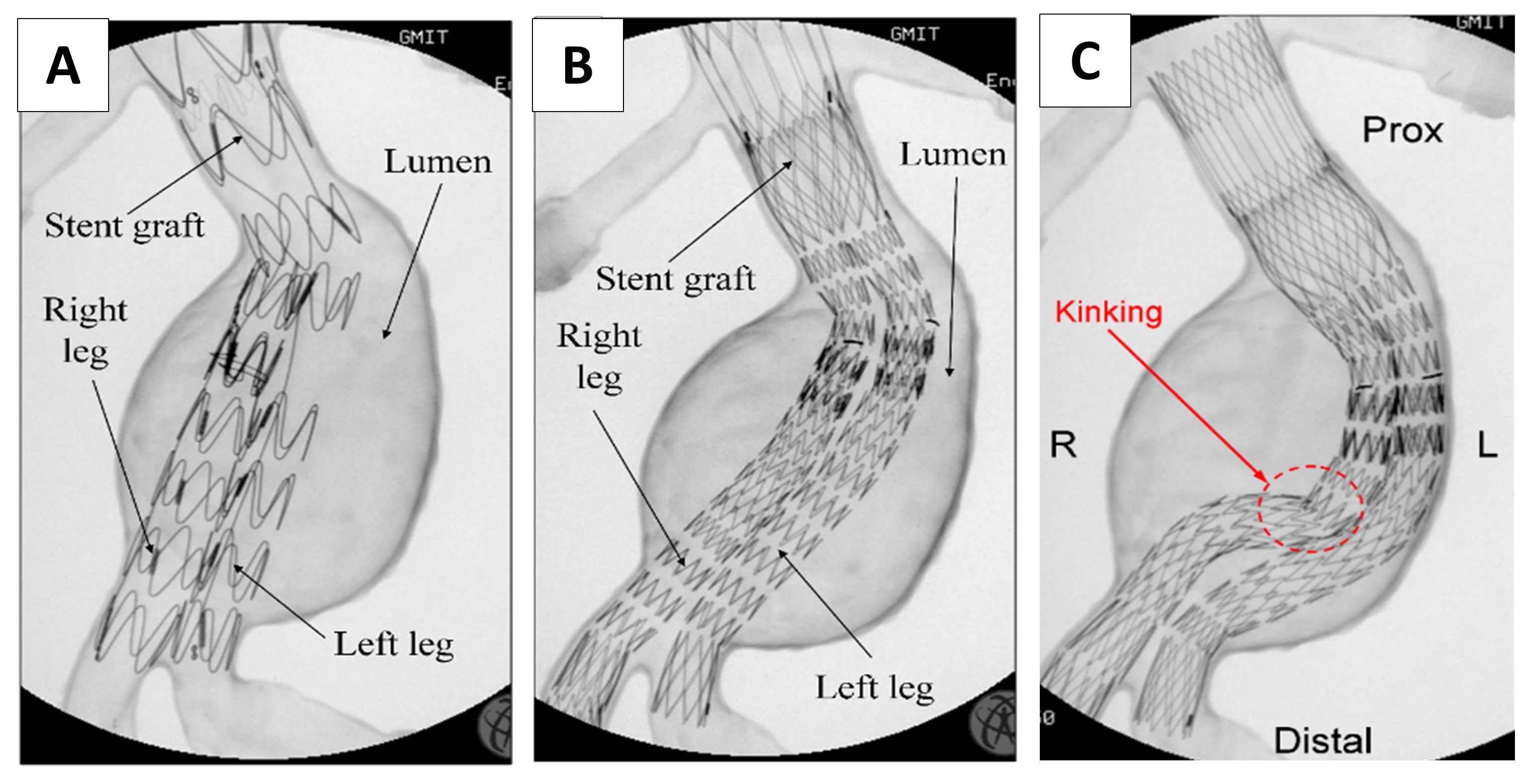
– Fluoroscope compatibility for delivery, deployment and retraction of bifurcated stent-grafts, balloon catheters for aortic occlusion and optimisation of deployment techniques to avoid stent kinking.
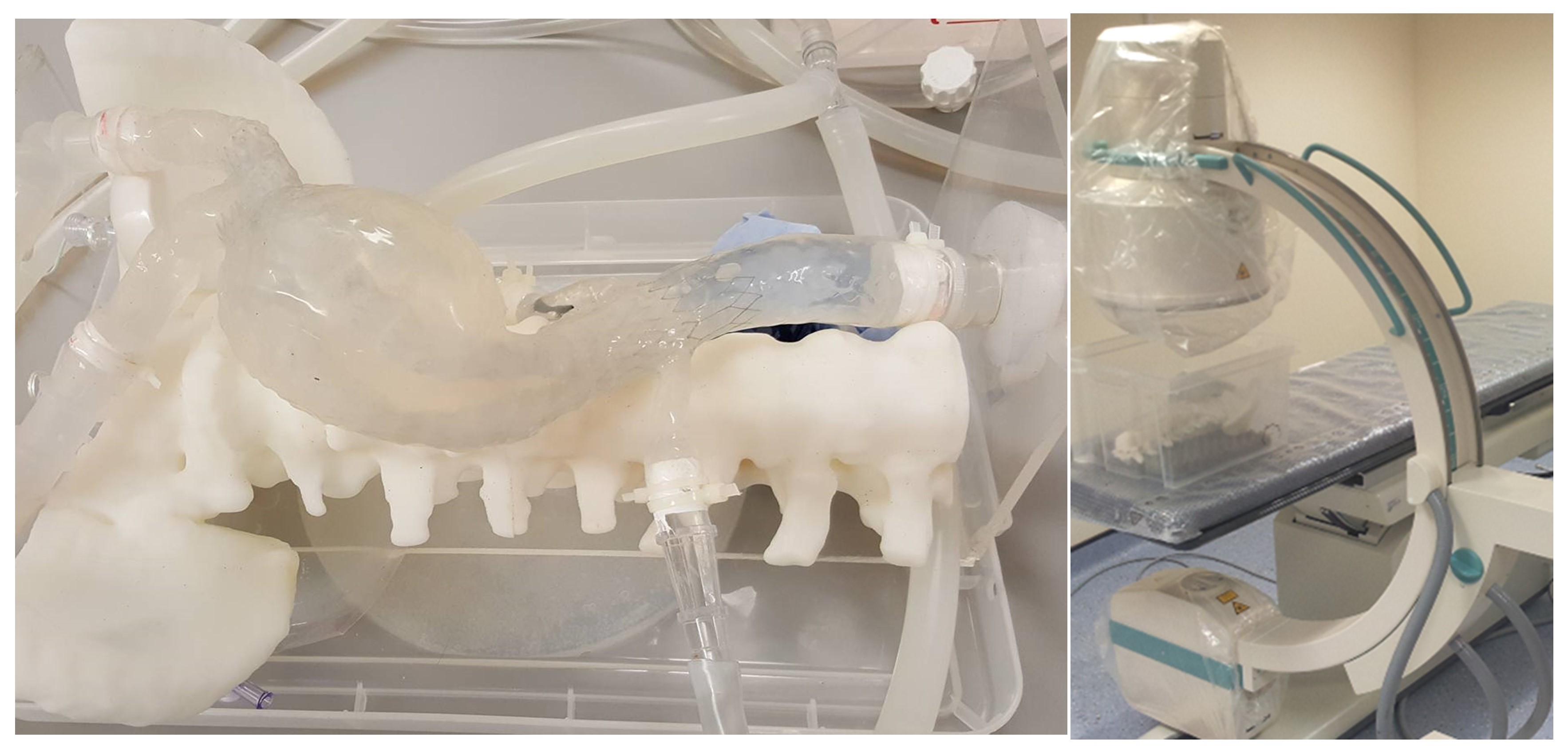
Stented AAA flexible model in-vitro fixture and C-arm.
Deployment test of two different stent-grafts under fluoroscopy (A and B). Resulted kinking during testing (C)
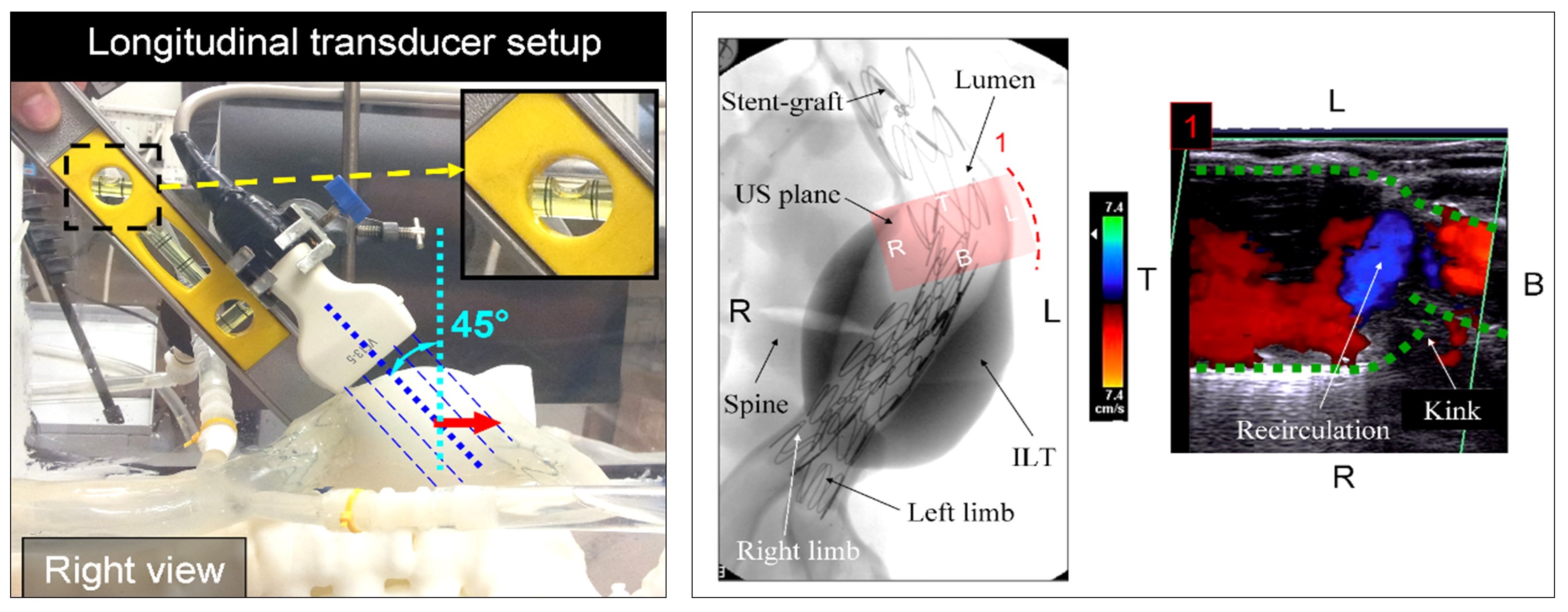
– Ultrasonic color Doppler imaging for deployment device resulted configuration and device impact on the blood flow.
Color-Doppler flow visualisation within the AAA stented model. Longitudinal transducer setup, (T – Top, B – Bottom, R – Right, L – Left).
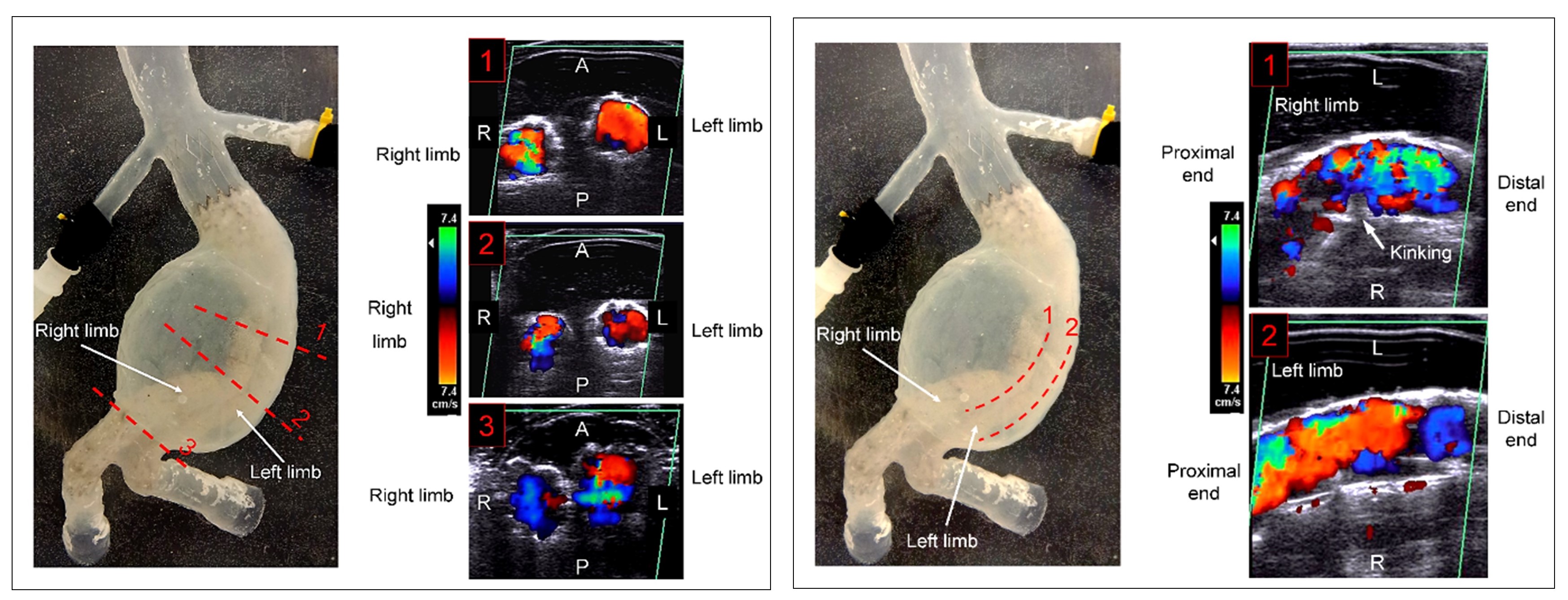
Color-Doppler ultrasound flow visualisation for 3 cross-sectional planes for the migrated stent-graft device, within the AAA model, (1) Section before the kinked region, (2) Section through the kinked region, (3) Section before the iliac bifurcation, (A – Anterior, P – Posterior, R – Right, L – Left).
The blood is replicated by a blood mimicking fluid prepared in house which replicates accurately the viscosity and density of the blood. The fluid is circulated through the in-vitro fixture by a versatile programmable BDC PD-1100 pump system accompanied by the StatysPD control and monitoring software. The pump system can achieve physiological hemodynamic simulation within in-vitro cardiovascular flow networks, where the PD-1100 acts as a pumping heart. With the PD-1100 and the appropriate components, the local hemodynamics of the heart or any vascular bed can be simulated. Typical applications include: cardiac valve evaluation and vascular studies of aortic, peripheral, carotid, femoral, venous, and pulmonary flow networks.
Project Benefits
MET Technology Gateway manages the projects with a customer focus to achieve traceable, reproducible and cost effective outputs. MET was able to provide the customer clinically relevant data informing the performance of the device and comparing data measured within the same system from commercially available devices.