Capsos Medical is a High Potential Start-Up medical device company based in Galway who design and devlop medical devices to penetrate total occlusion of blood vessels. The company has developed a patented balloon catheter and guidewire combination device called CapBuster to facilitate the treatment of chronic total occlusions (CTO). CapBuster re-opens the most resistant total occlusions where a calcified cap has formed on the surface of the blockage. This new device utilises standard tools and techniques used in in all angioplasties in every Cath lath in the world.
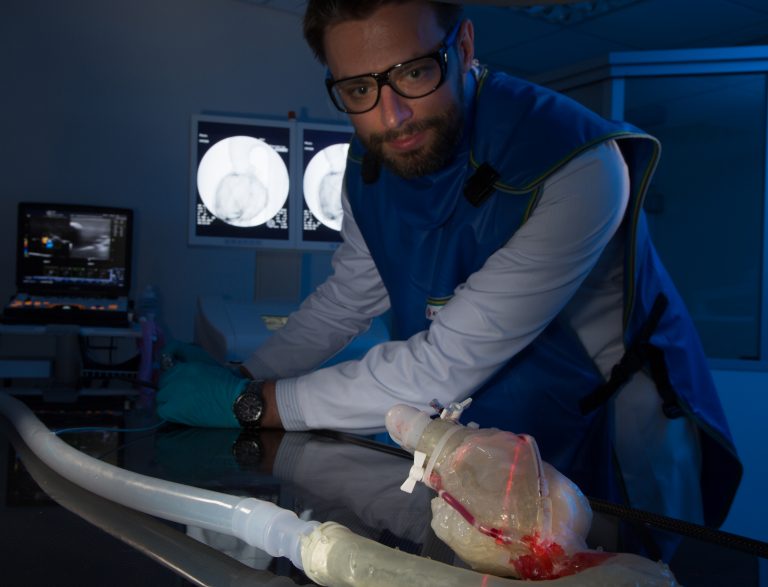
Currently, 50% of CTO’s are managed with medications, whilst approximately 40% are treated with bypass surgery, which is an invasive procedure with high surgical costs associated. No clinically relevant CTO’s was commercially available that replicates the specific anatomical challenges relevant to test the company’s device. MET Technology Gateway and Capsos Medical collaborated on an Enterprise Ireland innovation partnership funded project to address the technology gap by developing an in vitro simulation system for testing the performance of their product. MET researchers gathered the relevant clinical data and designed and developed various CTO’s plaque configurations, which were incorporated into clinically relevant coronary vessels. Following the vascular replication, MET designed a state-of-the-art customised in vitro simulation system with interchangeable vascular sections which was fluoroscope compatible.
By providing a highly realistic CTO model and a simulated use environment, the company could carry out design verification studies to evaluate and optimise their prototypes. This customised simulated system accelerated the product development cycle and reduced significant costs associated with pre-clinical animal testing. The capabilities developed through this project enabled Capsos Medical to design a CTO treatment device that performs in a fashion superior to other products on the market. Since completion of the project, GMIT has maintained a high level of interaction with the company and has generated various models with varying CTO properties.
“This project enabled Capsos to test our CTO treatment device (CapBuster), in an environment that accurately simulates challenging in-vivo conditions, and to evaluate and refine our designs rapidly and effectively, shortening the development cycle and therefore reducing the development cost.”
Brendan McLaughlin CEO Capsos Medical
This project was co-funded by the European Regional Development Fund and Enterprise Ireland under the Border, Midland and Western Regional Operational Programme 2014-2020.