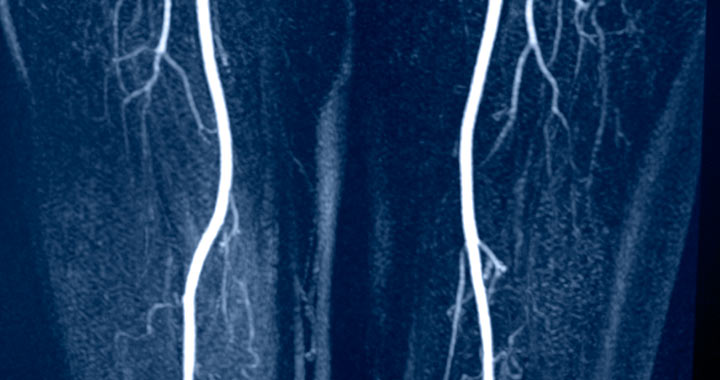
Cook Medical had designed a new delivery system which comprised of a new rotating thumbwheel system for the company’s Zilver®PTX drug eluting peripheral stent. The company had completed the testing of the device in-house and sought medical imaging technologies to verify the accuracy and ease of deployment prior to a first in man trial.
Using the company’s simulation system, GMIT engineers set up the medical imaging suite in the MET Gateway to allow the company’s engineers to conduct a series of deployment tests on its new device. MET provided the company all the data collated using the medical imaging technologies, accompanied by a copy of the equipment service and calibration records for the company’s technical file.
Following the completion of the design verification of the delivery system, the company quickly moved forward to carrying out a first in man trial in New Zealand. The new system, which is now available for purchase in Europe, US and Japan, was proven to provide simple deployment for a drug-eluting stent for the superficial femoral artery (SFA). Cook Medical R&D team continues to work with the MET Technology Gateway using the medical imaging suite on their next generation devices.